Safety and Immunogenicity of the mRNA-1273
Coronavirus Disease 2019 Vaccine in Solid Organ
Transplant Recipients
J Infect Dis. 2024 Mar 21:jiae140. doi: 10.1093/infdis/jiae140. Epub ahead of print. PMID: 38513368.
Background: Immunosuppressed individuals, including solid organ transplant recipients (SOTRs), are at high risk for severe COVID-19.
Methods: This open-label, phase 3b trial evaluated mRNA-1273 in 137 adult kidney and 77 liver SOTRs and 20 immunocompetent participants. In Part A, SOTRs received three 100-µg doses of mRNA-1273; immunocompetent participants received 2 doses. In Part B, an additional 100-µg dose was offered ≥4 months post-primary series. Here, we report interim trial results.
Results: mRNA-1273 was well-tolerated in SOTRs. Four serious adverse events were considered vaccine-related by the investigator in 3 SOTRs with pre-existing comorbidities. No vaccine-related biopsy-proven organ rejection events or deaths were reported. mRNA-1273 elicited modest neutralizing antibody (nAb) responses after dose 2 and improved responses after dose 3 in SOTRs. Post-dose 3 responses among liver SOTRs were comparable to post-dose 2 responses in immunocompetent participants. Post-additional dose responses were increased in SOTRs regardless of the primary series vaccination. In liver SOTRs, post-additional dose responses were ∼3-fold higher versus post-dose 2 but were lower than immunocompetent participant responses. Most kidney SOTRs received multiple immunosuppressants and had reduced antibody responses versus liver SOTRs.
Conclusions: mRNA-1273 (100 µg) was well-tolerated and dose 3 and the additional dose improved antibody responses among SOTRs.
A narrow T cell receptor repertoire instructs thymic differentiation of MHC class Ib-restricted CD8+ regulatory T cells
J Clin Invest. 2024 Jan 2;134(1):e170512. doi: 10.1172/JCI170512.
Although most CD8+ T cells are equipped to kill infected or transformed cells, a subset may regulate immune responses and preserve self-tolerance. Here, we describe a CD8 lineage that is instructed to differentiate into CD8 T regulatory cells (Tregs) by a surprisingly restricted set of T cell receptors (TCRs) that recognize MHC-E (mouse Qa-1) and several dominant self-peptides. Recognition and elimination of pathogenic target cells that express these Qa-1-self-peptide complexes selectively inhibits pathogenic antibody responses without generalized immune suppression. Immunization with synthetic agonist peptides that mobilize CD8 Tregs in vivo efficiently inhibit antigraft antibody responses and markedly prolong heart and kidney organ graft survival. Definition of TCR-dependent differentiation and target recognition by this lineage of CD8 Tregs may open the way to new therapeutic approaches to inhibit pathogenic antibody responses.
Uncovering a novel role of focal adhesion and interferon-gamma in cellular rejection of kidney allografts at single cell resolution
Front Immunol. 2023 Mar 31:14:1139358. doi: 10.3389/fimmu.2023.1139358. eCollection 2023.
Background: Kidney transplant recipients are currently treated with nonspecific immunosuppressants that cause severe systemic side effects. Current immunosuppressants were developed based on their effect on T-cell activation rather than the underlying mechanisms driving alloimmune responses. Thus, understanding the role of the intragraft microenvironment will help us identify more directed therapies with lower side effects.
Methods: To understand the role of the alloimmune response and the intragraft microenvironment in cellular rejection progression, we conducted a Single nucleus RNA sequencing (snRNA-seq) on one human non-rejecting kidney allograft sample, one borderline sample, and T-cell mediated rejection (TCMR) sample (Banff IIa). We studied the differential gene expression and enriched pathways in different conditions, in addition to ligand-receptor (L-R) interactions.
Results: Pathway analysis of T-cells in borderline sample showed enrichment for allograft rejection pathway, suggesting that the borderline sample reflects an early rejection. Hence, this allows for studying the early stages of cellular rejection. Moreover, we showed that focal adhesion (FA), IFNg pathways, and endomucin (EMCN) were significantly upregulated in endothelial cell clusters (ECs) of borderline compared to ECs TCMR. Furthermore, we found that pericytes in TCMR seem to favor endothelial permeability compared to borderline. Similarly, T-cells interaction with ECs in borderline differs from TCMR by involving DAMPS-TLRs interactions.
Conclusion: Our data revealed novel roles of T-cells, ECs, and pericytes in cellular rejection progression, providing new clues on the pathophysiology of allograft rejection.
Chimeric antigen receptor Treg therapy in transplantation
Trends Immunol. 2024 Jan;45(1):48-61. doi: 10.1016/j.it.2023.11.005. Epub 2023 Dec 19.
In the quest for more precise and effective organ transplantation therapies, chimeric antigen receptor (CAR) regulatory T cell (Treg) therapies represent a potential cutting-edge advance. This review comprehensively analyses CAR Tregs and how they may address important drawbacks of polyclonal Tregs and conventional immunosuppressants. We examine a growing body of preclinical findings of CAR Treg therapy in transplantation, discuss CAR Treg design specifics, and explore established and attractive new targets in transplantation. In addition, we explore present impediments where future studies will be necessary to determine the efficacy of CAR Tregs in reshaping alloimmune responses and transplant microenvironments to reduce reliance on chemical immunosuppressants. Overall, ongoing studies and trials are crucial for understanding the full scope of CAR Treg therapy in transplantation.
High-Throughput/High Content Imaging Screen Identifies Novel Small Molecule Inhibitors and Immunoproteasomes as Therapeutic Targets for Chordoma
Pharmaceutics. 2023 Apr 18;15(4):1274. doi: 10.3390/pharmaceutics15041274.
Chordomas account for approximately 1-4% of all malignant bone tumors and 20% of primary tumors of the spinal column. It is a rare disease, with an incidence estimated to be approximately 1 per 1,000,000 people. The underlying causative mechanism of chordoma is unknown, which makes it challenging to treat. Chordomas have been linked to the T-box transcription factor T (TBXT) gene located on chromosome 6. The TBXT gene encodes a protein transcription factor TBXT, or brachyury homolog. Currently, there is no approved targeted therapy for chordoma. Here, we performed a small molecule screening to identify small chemical molecules and therapeutic targets for treating chordoma. We screened 3730 unique compounds and selected 50 potential hits. The top three hits were Ribociclib, Ingenol-3-angelate, and Duvelisib. Among the top 10 hits, we found a novel class of small molecules, including proteasomal inhibitors, as promising molecules that reduce the proliferation of human chordoma cells. Furthermore, we discovered that proteasomal subunits PSMB5 and PSMB8 are increased in human chordoma cell lines U-CH1 and U-CH2, confirming that the proteasome may serve as a molecular target whose specific inhibition may lead to better therapeutic strategies for chordoma.
Discovery and Validation of a Urinary Exosome mRNA Signature for the Diagnosis of Human Kidney Transplant Rejection
Background Developing a noninvasive clinical test to accurately diagnose kidney allograft rejection is critical to improve allograft outcomes. Urinary exosomes, tiny vesicles released into the urine that carry parent cells’ proteins and nucleic acids, reflect the biologic function of the parent cells within the kidney, including immune cells. Their stability in urine makes them a potentially powerful tool for liquid biopsy and a noninvasive diagnostic biomarker for kidney-transplant rejection.
Methods Using 192 of 220 urine samples with matched biopsy samples from 175 patients who underwent a clinically indicated kidney-transplant biopsy, we isolated urinary exosomal mRNAs and developed rejection signatures on the basis of differential gene expression. We used crossvalidation to assess the performance of the signatures on multiple data subsets.
Results An exosomal mRNA signature discriminated between biopsy samples from patients with all-cause rejection and those with no rejection, yielding an area under the curve (AUC) of 0.93 (95% CI, 0.87 to 0.98), which is significantly better than the current standard of care (increase in eGFR AUC of 0.57; 95% CI, 0.49 to 0.65). The exosome-based signature’s negative predictive value was 93.3% and its positive predictive value was 86.2%. Using the same approach, we identified an additional gene signature that discriminated patients with T cell–mediated rejection from those with antibody-mediated rejection (with an AUC of 0.87; 95% CI, 0.76 to 0.97). This signature’s negative predictive value was 90.6% and its positive predictive value was 77.8%.
Conclusions Our findings show that mRNA signatures derived from urinary exosomes represent a powerful and noninvasive tool to screen for kidney allograft rejection. This finding has the potential to assist clinicians in therapeutic decision making.
Regulatory T cells engineered with TCR signaling–responsive IL-2 nanogels suppress alloimmunity in sites of antigen encounter
Science translational medicine vol. 12,569 (2020): eaaw4744. doi:10.1126/scitranslmed.aaw4744
Microneedle-Based Local Delivery of CCL22 and IL-2 Enriches Treg Homing to the Skin Allograft and Enables Temporal Monitoring of Immunotherapy Efficacy
Adv. Funct. Mater. 2021, 31, 2100128. https://doi.org/10.1002/adfm.202100128
Donor myeloid derived suppressor cells (MDSCs) prolong allogeneic cardiac graft survival through programming of recipient myeloid cells in vivo
Sci Rep 10, 14249 (2020). https://doi.org/10.1038/s41598-020-71289-z
Regulatory CD8 T cells that recognize Qa-1 expressed by CD4 T-helper cells inhibit rejection of heart allografts
Proc Natl Acad Sci U S A. 2020 Mar 17;117(11):6042-6046. doi: 10.1073/pnas.1918950117.
Blocking IFNAR1 inhibits multiple myeloma-driven Treg expansion and immunosuppression
J Clin Invest. 2018 Jun 1;128(6):2487-2499. doi: 10.1172/JCI88169.
Despite significant advances in the treatment of multiple myeloma (MM), most patients succumb to disease progression. One of the major immunosuppressive mechanisms that is believed to play a role in myeloma progression is the expansion of regulatory T cells (Tregs). In this study, we demonstrate that myeloma cells drive Treg expansion and activation by secreting type 1 interferon (IFN). Blocking IFN α and β receptor 1 (IFNAR1) on Tregs significantly decreases both myeloma-associated Treg immunosuppressive function and myeloma progression. Using syngeneic transplantable murine myeloma models and bone marrow (BM) aspirates of MM patients, we found that Tregs were expanded and activated in the BM microenvironment at early stages of myeloma development. Selective depletion of Tregs led to a complete remission and prolonged survival in mice injected with myeloma cells. Further analysis of the interaction between myeloma cells and Tregs using gene sequencing and enrichment analysis uncovered a feedback loop, wherein myeloma-cell-secreted type 1 IFN induced proliferation and expansion of Tregs. By using IFNAR1-blocking antibody treatment and IFNAR1-knockout Tregs, we demonstrated a significant decrease in myeloma-associated Treg proliferation, which was associated with longer survival of myeloma-injected mice. Our results thus suggest that blocking type 1 IFN signaling represents a potential strategy to target immunosuppressive Treg function in MM.
Human regulatory T cells undergo self-inflicted damage via granzyme pathways upon activation
JCI Insight. 2017 Nov 2;2(21). pii: 91599. doi: 10.1172/jci.insight.91599.
Tregs hold great promise as a cellular therapy for multiple immunologically mediated diseases, given their ability to control immune responses. The success of such strategies depends on the expansion of healthy, suppressive Tregs ex vivo and in vivo following the transfer. In clinical studies, levels of transferred Tregs decline sharply in the blood within a few days of the transfer. Tregs have a high rate of apoptosis. Here, we describe a new mechanism of Treg self-inflicted damage. We show that granzymes A and -B (GrA and GrB), which are highly upregulated in human Tregs upon stimulation, leak out of cytotoxic granules to induce cleavage of cytoplasmic and nuclear substrates, precipitating apoptosis in target cells. GrA and GrB substrates were protected from cleavage by inhibiting granzyme activity in vitro. Additionally, we show – by using cytometry by time of flight (CYTOF) – an increase in GrB-expressing Tregs in the peripheral blood and renal allografts of transplant recipients undergoing rejection. These GrB-expressing Tregs showed an activated phenotype but were significantly more apoptotic than non-GrB expressing Tregs. This potentially novel finding improves our understanding of Treg survival and suggests that manipulating Gr expression or activity might be useful for designing more effective Treg therapies.
Integrated Kidney Exosome Analysis for the Detection of Kidney Transplant Rejection
ACS Nano. 2017 Nov 28;11(11):11041-11046. doi: 10.1021/acsnano.7b05083. Epub 2017 Oct 25.
Kidney transplant patients require life-long surveillance to detect allograft rejection. Repeated biopsy, albeit the clinical gold standard, is an invasive procedure with the risk of complications and comparatively high cost. Conversely, serum creatinine or urinary proteins are noninvasive alternatives but are late markers with low specificity. We report a urine-based platform to detect kidney transplant rejection. Termed iKEA (integrated kidney exosome analysis), the approach detects extracellular vesicles (EVs) released by immune cells into urine; we reasoned that T cells, attacking kidney allografts, would shed EVs, which in turn can be used as a surrogate marker for inflammation. We optimized iKEA to detect T-cell-derived EVs and implemented a portable sensing system. When applied to clinical urine samples, iKEA revealed high level of CD3-positive EVs in kidney rejection patients and achieved high detection accuracy (91.1%). Fast, noninvasive, and cost-effective, iKEA could offer new opportunities in managing transplant recipients, perhaps even in a home setting.
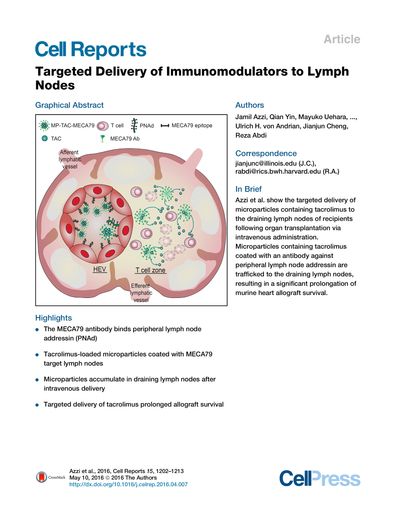
Targeted Delivery of Immunomodulators to Lymph Nodes
Cell Rep. 2016 May 10;15(6):1202-13. doi: 10.1016/j.celrep.2016.04.007. Epub 2016 Apr 28.
Active-targeted delivery to lymph nodes represents a major advance toward more effective treatment of immune-mediated disease. The MECA79 antibody recognizes peripheral node addressin molecules expressed by high endothelial venules of lymph nodes. By mimicking lymphocyte trafficking to the lymph nodes, we have engineered MECA79-coated microparticles containing an immunosuppressive medication, tacrolimus. Following intravenous administration, MECA79-bearing particles showed marked accumulation in the draining lymph nodes of transplanted animals. Using an allograft heart transplant model, we show that targeted lymph node delivery of microparticles containing tacrolimus can prolong heart allograft survival with negligible changes in tacrolimus serum level. Using MECA79 conjugation, we have demonstrated targeted delivery of tacrolimus to the lymph nodes following systemic administration, with the capacity for immune modulation in vivo.
Structure of human immunoproteasome with a reversible and noncompetitive inhibitor
Nat Commun. 2017 Nov 22;8(1):1692. doi: 10.1038/s41467-017-01760-5.
Proteasome inhibitors benefit patients with multiple myeloma and B cell-dependent autoimmune disorders but exert toxicity from inhibition of proteasomes in other cells. Toxicity should be minimized by reversible inhibition of the immunoproteasome β5i subunit while sparing the constitutive β5c subunit. Here we report β5i-selective inhibition by asparagine-ethylenediamine (AsnEDA)-based compounds and present the high-resolution cryo-EM structural analysis of the human immunoproteasome. Despite inhibiting noncompetitively, an AsnEDA inhibitor binds the active site. Hydrophobic interactions are accompanied by hydrogen bonding with β5i and β6 subunits. The inhibitors are far more cytotoxic for myeloma and lymphoma cell lines than for hepatocarcinoma or non-activated lymphocytes. They block human B-cell proliferation and promote apoptotic cell death selectively in antibody-secreting B cells, and to a lesser extent in activated human T cells. Reversible, β5i-selective inhibitors may be useful for treatment of diseases involving activated or neoplastic B cells or activated T cells.
- Zhan W, Li D, Saha P, Wang R, Zhang H, Ajay AK, Deban C, Sukenick G, Azzi J, Lin G. Discovery of Highly Selective Inhibitors of the Human Constitutive Proteasome ß5c Chymotryptic Subunit. J Med Chem. 2023 01 26; 66(2):1172-1185. PMID: 36608337.
- Eskandari SK, Allos H, Al Dulaijan BS, Melhem G, Sulkaj I, Alhaddad JB, Saad AJ, Deban C, Chu P, Choi JY, Kollar B, Pomahac B, Riella LV, Berger SP, Sanders JSF, Lieberman J, Li L, Azzi JR. mTORC1 Inhibition Protects Human Regulatory T Cells From Granzyme-B-Induced Apoptosis. Front Immunol. 2022; 13:899975. PMID: 35757726; PMCID: PMC9229986.
- Al Jurdi A, Gassen RB, Borges TJ, Lape IT, Morena L, Efe O, Solhjou Z, El Fekih R, Deban C, Bohan B, Pattanayak V, Kotton CN, Azzi JR, Riella LV. Suboptimal antibody response against SARS-CoV-2 Omicron variant after third dose of mRNA vaccine in kidney transplant recipients. Kidney Int. 2022 06; 101(6):1282-1286. PMID: 35429496; PMCID: PMC9006415.
- Zavidij O, Haradhvala NJ, Mouhieddine TH, Sklavenitis-Pistofidis R, Cai S, Reidy M, Rahmat M, Flaifel A, Ferland B, Su NK, Agius MP, Park J, Manier S, Bustoros M, Huynh D, Capelletti M, Berrios B, Liu CJ, He MX, Braggio E, Fonseca R, Maruvka Y, Guerriero JL, Goldman M, van Allen E, McCarroll SA, Azzi J, Getz G, Ghobrial IM. Single-cell RNA sequencing reveals compromised immune microenvironment in precursor stages of multiple myeloma. Nat Cancer. 2020 May; 1(5):493-506. PMID: 33409501.
- Ordikhani F, Uehara M, Kasinath V, Dai L, Eskandari SK, Bahmani B, Yonar M, Azzi JR, Haik Y, Sage PT, Murphy GF, Annabi N, Schatton T, Guleria I, Abdi R. Targeting antigen-presenting cells by anti-PD-1 nanoparticles augments antitumor immunity. JCI Insight. 2018 Oct 18;3(20). PubMed PMID: 30333312.
- Bahmani B, Uehara M, Jiang L, Ordikhani F, Banouni N, Ichimura T, Solhjou Z, Furtmüller GJ, Brandacher G, Alvarez D, von Andrian UH, Uchimura K, Xu Q, Vohra I, Yilmam OA, Haik Y, Azzi JR, Kasinath V, Bromberg JS, McGrath MM, Abdi R. Targeted delivery of immune therapeutics to lymph nodes prolongs cardiac allograft survival. J Clin Invest. 2018 Oct 2. PubMed PMID: 30277476.
- Borges TJ, Murakami N, Machado FD, Murshid A, Lang BJ, Lopes RL, Bellan LM, Uehara M, Antunes KH, Pérez-Saéz MJ, Birrane G, Vianna P, Gonçalves JIB, Zanin RF, Azzi JR, Abdi R, Ishido S, Shin JS, Souza APD, Calderwood SK, Riella LV, Bonorino C. March1-dependent modulation of donor MHC II on CD103(+) dendritic cells mitigates alloimmunity. Nat Commun. 2018 Aug 28;9(1):3482. PubMed PMID: 30154416; PMC6113260.
- Kurdi AT, Glavey SV, Bezman NA, Jhatakia A, Guerriero JL, Manier S, Moschetta M, Mishima Y, Roccaro A, Detappe A, Liu CJ, Sacco A, Huynh D, Tai YT, Robbins MD, Azzi JR, Ghobrial IM. Antibody-Dependent Cellular Phagocytosis by Macrophages is a Novel Mechanism of Action of Elotuzumab. Mol Cancer Ther. 2018 Jul;17(7):1454-63. PubMedPMID: 29654064; PMC6030488.
- Kawano Y, Zavidij O, Park J, Moschetta M, Kokubun K, Mouhieddine TH, Manier S, Mishima Y, Murakami N, Bustoros M, Pistofidis RS, Reidy M, Shen YJ, Rahmat M, Lukyanchykov P, Karreci ES, Tsukamoto S, Shi J, Takagi S, Huynh D, Sacco A, Tai YT, Chesi M, Bergsagel PL, Roccaro AM, Azzi JR, Ghobrial IM. Blocking IFNAR1 inhibits multiple myeloma-driven Treg expansion and immunosuppression. J Clin Invest. 2018 Jun 1;128(6):2487-99. PubMed PMID: 29558366; PMC5983341.